


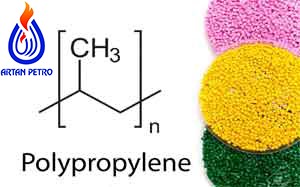
Polypropylene (PP) is a highly crystalline thermoplastic resin that is formed by a chain. Polymerization of propylene (CH 2 = CHCH 3), a compound gas is obtained.
By thermal cracking of ethane, propane, butane, or naphtha fraction oil. The polymer repeater unit has the following structure:
Only the isotactic form of polypropylene is marketed in significant quantities.
(In isotactic polypropylene, [CH 3] all methyl groups are located along one side of the polymer chain.) At low temperatures and pressures using
Produced from Ziegler-Nata catalysts.
Polypropylene has some of the properties of polyethylene, but it is harder, has a higher melting point, and is less susceptible to oxidation.
Most of it is dedicated to fibers, where the main material is fabric for home furniture such as upholstery and outdoor carpets.
There are also several industrial end uses for polypropylene fibers, including rope and cord.
Disposable non-woven fabrics are used for diapers and medical applications, and non-woven fabrics are used for stabilization.
Use land and reinforcement in construction and road construction.
However, polypropylene fiber is not important due to its very low moisture absorption, limited pigmentation and low softening point (an important factor when ironing clothes).
As a plastic, polypropylene is used as a shock in food bottles, shampoos and other household liquids.
Also to many products such as appliance compartments, dishwasher utensils, toys, shells
Car batteries and outdoor furniture are molded by injection. When a thin section of polypropylene is molded
It bends repeatedly, forming a molecular structure.
Which is able to withstand extra flexibility without fail.
This resistance to fatigue leads to the design of polypropylene boxes and other containers with hinged covers.
It is generally accepted that isotactic polypropylene was discovered in 1954 by an Italian chemist.
Julio Nata and his assistant Paolo Cheney, in collaboration with Montecatini (now Montedison SpA) and the use of catalysts of this type
It was recently invented by Karl Ziegler to synthesize polyethylene.
(Part of the recognition of this achievement was the 1963 Nobel Prize in Chemistry with Ziegler.
Commercial production of polypropylene was started by Hercules Incorporated, Montecatini and Farbwerke Hoechst AG Germany in 1957.
Production and consumption since the early 1980s due to the invention of more efficient catalyst systems by Montedison and Mitsui & Co. Ltd.
Japanese increases significantly.
This thermoplastic resin is a rigid and relatively brittle polymer of styrene (CH 2 = CHC 6 H 5).
Styrene, also called phenylethylene, is obtained by reacting ethylene with benzene in the presence of aluminum chloride to produce ethylene benzene.
Which is then dehydrated to obtain a clear and liquid styrene.
Styrene monomer using free radical scavengers is primarily used in bulk and polymer suspension processes, although
Solution and emulsion methods are also used.
The presence of a phenyl group pendant (C 6 H 5) is the key to the properties of polystyrene.
These large, ring-shaped groups prevent the closure of polymer chains, both close and crystalline, respectively.
So that solid polystyrene is transparent.
In addition, phenyl rings restrict the rotation of the chains around carbon-carbon bonds
Therefore, they give significant resistance to the polymer.

Styrenase polymerization became known in 1839, when German pharmacist Edward Simon reported its conversion to solid styrene
It was later renamed Metastyrol.
In the late 1930s, little commercial use of this polymer was found due to its fragility and insanity (minute cracking), which resulted in
It was the impurities that caused the polymer chains to cross-link.
By 1937, Robert Dreisbach and others purified the monomer in the physics laboratory of the Dow Chemical Company, an experimental factory process.
Created for this polymer, which was produced commercially in 1938.
Foamedpolystyrene in insulating containers, packaging and foodstuffs such as beverage cups
They make egg cartons and disposable plates and trays.
Solid polystyrene products include injection molded food containers, audio cassette holders and packaging items
Compact discs.
Many foods are packed in clear polystyrene trays due to their high permeability to gas and good transfer of water vapor from the material.
Production of General purpose polystyrene with different grades

info@artanpetro.com
Qom Shokouhieh Industrial Town, end of the second phase, Babaei Square, Babaei St., Alam al-Huda St. 1, No. 1331
+982533346396![]()
+982533346473![]()