


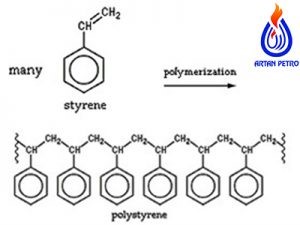
Polystyrene, hard, hard, shiny and transparent synthetic resin is produced by polymerization of polystyrene.
Widely used in the food service industry as rigid trays and utensils, disposable tableware and glasses, plates and floor bowls.
They also copolymer or mix with other polymers and lend themselves to a number of
Lend important plastic and rubber products.
Styrene is obtained by reacting ethylene with benzene in the presence of aluminum chloride to produce ethylene benzene.
The benzene group in this compound is then functionalized to phenylethylene and styrene, clear dehydrogenated hydrocarbon liquid with CH structure
Chemical 2 = CHC 6 H 5. Styrene is used primarily in bulk and polymer suspension processes using free radical scavengers.
However, solution and emulsion methods are also used.
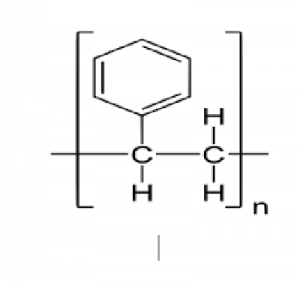
The presence of a phenyl group pendant (C 6 H 5) is the key to the properties of polystyrene.
Solid polystyrene is transparent due to the presence of these large ring-shaped molecular groups that close
Prevents polymer chains in close and crystal arrangements.
In addition, phenyl rings limit the rotation of the chains around the carbon-carbon bonds and give the polymer its remarkable strength.
Polymerization of styrene has been known since 1839, when German pharmacist Edward Simon converted it to a solid.
Which was later renamed metastyrol, he reported.
In the late 1930s, little use was made of the polymer due to its fragility and insanity (minute cracking).
Which was caused by impurities that caused the polymer chains to cross-link.
In 1937, American chemist Robert Dreisbach and others at the Dow Chemical Company acquired pure styrene laboratory physics.
Monomerase was developed by ethylbenzene dehydrogenation and an experimental polymerization process.
.
Due to the low cost of producing large quantities of styrene monomer, ease of polymer formation that melts in operation
Injection molding and optical and physical properties of materials, this material quickly became one of the most important modern plastics.
In the past, this floor was made with the help of chlorofluorocarbon blowing agents – a group of compounds that were used to make this foam in the past with the help of chlorofluorocarbon blowing agents – a group of compounds that
Prohibited for environmental reasons.
Now foamed by pentane or carbon dioxide, into insulating and packaging materials
They also make food containers such as drink glasses, egg cartons, disposable plates and trays.
Solid polystyrene products include injection molded food containers, video cassettes and audio cassettes, and items for
Audio cassettes and compact discs
Many fresh foods are packed in polystyrene trays with a smooth vacuum due to their high permeability to gas and good transfer of water vapor from the material.
Bright windows in many postal envelopes are made of polystyrene film.
Polystyreneplastic recycling code number is # 6.
These recycled products are usually melted down and used in floor insulation.
It is also fragile and flammable despite its beneficial properties.
It also softens in boiling water and turns yellow after prolonged exposure to sunlight, without the addition of chemical stabilizers.
In order to reduce brittleness and improve impact resistance, more than half of the total polystyrene produced is mixed with 5 to 10% butadiene rubber.
This compound, suitable for toys and home appliance parts, is marketed as High Impact Polystyrene (HIPS).
Production of General purpose polystyrene with different grades

info@artanpetro.com
Qom Shokouhieh Industrial Town, end of the second phase, Babaei Square, Babaei St., Alam al-Huda St. 1, No. 1331
+982533346396![]()
+982533346473![]()